Diseases
Click here to view more Diseases
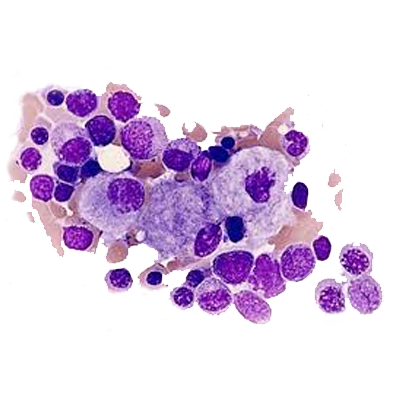
Gaucher disease (GD) is a sphingolipidoses, a subgroup of lysosomal storage diseases. It is the most common lysosomal storage disease, having an autosomal recessive inheritance and resulting from a deficiency of the glucocerebrosidase enzyme due to a genetic defect on the glucosylceramidase beta (GBA1) gene. The enzyme deficiency results in the inability to break down glucosylceramide in lysosomes, and lipid-laden macrophages accumulate in the tissues.
Gaucher disease (GD) is the commonest lysosomal storage disorder (LSD) with an estimated global incidence of 1: 40,000 to 1:60,000 live births. In the Ashkenazi (Eastern European) Jewish population, with a carrier frequency of 6% compared to 0.7% to 0.8% of the non-Jewish population. Gaucher disease's exact prevalence in India is not fully documented due to a lack of centralized registries, due to the tradition of consanguineous marriages in parts of the country, it seems likely that the frequency of Gaucher disease may be higher in India. The most common form of Gaucher disease is type 1, which has a very variable phenotype ranging from early childhood symptoms to no symptoms throughout life but typically does not have a neurological component. In contrast to type 1, types 2 and 3 are rare and affect the central nervous system.
Glucosylceramide, the accumulated glycolipid, is primarily derived from the phagocytosis and degradation of senescent leukocytes and erythrocyte membranes. The glycolipid storage gives rise to the characteristic Gaucher cells, macrophages engorged with lipid with a crumpled–tissue-paper appearance and displaced nuclei. The factors that contribute to neurologic involvement in patients with types 2 and 3 disease are still unknown but may be related to the accumulation of a cytotoxic glycolipid, glucosylsphingosine, in the brain due to the severe deficiency of glucocerebrosidase activity or to neuroinflammation. Glucosylceramide accumulation in the bone marrow, liver, spleen, lungs, and other organs contributes to pancytopenia, massive hepatosplenomegaly, and, at times, diffuse infiltrative pulmonary disease. Progressive infiltration of Gaucher cells in the bone marrow may lead to thinning of the cortex, pathologic fractures, bone pain, bony infarcts, and osteopenia.
Individuals with Gaucher disease may exhibit the following signs and symptoms:
The diagnosis of Gaucher disease depends upon finding a low GBA1 enzyme level in peripheral blood leukocytes and establishing the presence of mutant alleles in the GBA1 gene. Even though only a blood sample is needed to diagnose Gaucher disease, some patients undergo unnecessary invasive bone marrow or liver biopsy before making a correct diagnosis
| Indication | Dose | Contraindications | Adverse Effects |
|---|---|---|---|
| Eliglustat is a glucosylceramide synthase inhibitor indicated for the long-term treatment of adult patients with Gaucher disease type 1 who are CYP2D6 extensive metabolizers (EMs), intermediate metabolizers(IMs), or poor metabolizers (PMs) as detected by an FDA-cleared test. | EMs and IMs: 84 mg orally twice daily. PMs: 84 mg orally once daily. | Taking a strong or moderate CYP2D6 inhibitor concomitantly with a strong or moderate CYP3A inhibitor. | Most common adverse reactions (≥10%) are: fatigue, headache, nausea, diarrhea, back pain, pain in extremities, and upper abdominal pain. |
New innovations in Gaucher disease treatment include gene therapy, brain-targeted therapies, and small molecule chaperones, offering potential for long-term benefits and reducing the need for continuous enzyme replacement therapy. Additionally, research focuses on improving current enzyme replacement therapies (ERT) with technologies like nanoERT and exploring new delivery methods like intranasal routes for brain delivery.
Gaucher disease, a lysosomal storage disorder, presents significant challenges in diagnosis, treatment, and long-term management, particularly for the more severe forms. While enzyme replacement therapy (ERT) has revolutionized the management of Type 1 Gaucher disease, challenges remain with its cost, delivery limitations, and potential for antibody development. Future directions focus on developing targeted therapies, addressing the neurological complications of Type 3, and ensuring equitable access to treatment, especially in resource-poor countries.